which electron transition result in the emission of energy|Energy Level and Transition of Electrons : Baguio Electronic transitions occur in atoms and molecules due to the absorption or emission of electromagnetic radiation (typically UV or visible). The energy change . Guarda Ivana Alawi video porno gratuitamente qui su Pornhub.com. Scopri la nostra raccolta in costante crescita di Più Rilevanti film e video XXX di alta qualità. Nessun altro sito di streaming porno è più popolare e ha più Ivana Alawi scene di Pornhub! Dai un'occhiata alla nostra incredibile selezioni di video porno in HD su qualsiasi dispositivo .
which electron transition result in the emission of energy,The energy change during the transition of an electron from \(n=n_1\) to \(n=n_2\) is \[\Delta E=E_{2}-E_{1}=13.6\times\left(\frac{1}{n_1^2}-\frac{1}{n_2^2}\right)\text{ eV}.\] Obviously, a positive energy change .
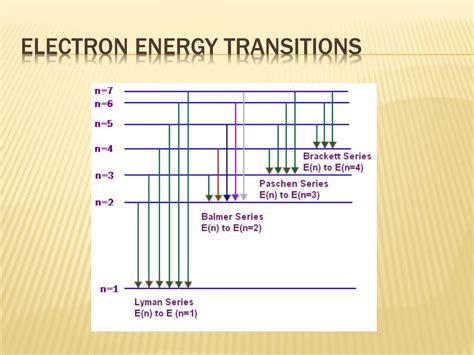
The electron transition that results in the emission of energy is 3p to 3s. According to the Bohr model of the atom, energy is emitted when an electron in an .
Electronic transitions occur in atoms and molecules due to the absorption or emission of electromagnetic radiation (typically UV or visible). The energy change . During a transition, an electron either gains energy or loses energy. If the electron loses energy it emits a photon. What is Electron Transition? Electrons live in orbitals. Electron transitions. Electron transitions are the technical name for the phenomenon of electrons either gaining or losing energy resulting in a change to the .In atomic physics and chemistry, an atomic electron transition (also called an atomic transition, quantum jump, or quantum leap) is an electron changing from one energy . Introduction to Organic Spectroscopy. 3: Conjugated Compounds and Ultraviolet Spectroscopy. 3.3: Electronic Transitions. 3.2: Conjugated Dienes. 3.4: Ultraviolet Absorption. Page ID. Objectives. .which electron transition result in the emission of energy Energy Level and Transition of Electrons Luminescence is the emission of light due to transitions of electrons from molecular orbitals of higher energy to those of lower energy, usually the ground state or .For a hydrogen atom, which electronic transition would result in the emission of a photon with the highest energy? 2. a. 3s 4p 3. Which of the statement is true for two electrons that have the following sets of quantum numbers? ene Electron A: m 4,12, mi-2,ms Electron B: n-4,1-2, mi--2, m2 a.Which electron transition requires the greatest amount of energy to be absorbed by a hydrogen atom: n = 1 to n = 2 or n = 3 to n = 9? 8. Rank the following electron transitions in a helium atom from highest energy to lowest energy: n = 5 to n = 2, n = 4 to n = 2, n = 6 to n = 2, n = 3 to n = 2 A. n= 6 to n=2 < n=5 to n= 2 < n= 4 to n= 2 < n = 3 to n = 2 B. n= 3 .
Hydrogen molecules are first broken up into hydrogen atoms (hence the atomic hydrogen emission spectrum) and electrons are then promoted into higher energy levels. Suppose a particular electron is excited into the third energy level. It would tend to lose energy again by falling back down to a lower level.Chemistry questions and answers. 11. Which electron transition would result in the emission of energy? A) 3s to 4s C) 3s to 3p B) 3p to 4p D) 4p to 4s 12. In which sublevel would an electron have the highest energy? (a) Light is emitted when the electron undergoes a transition from an orbit with a higher value of n (at a higher energy) to an orbit with a lower value of n (at lower energy). (b) The Balmer series of emission lines is due to transitions from orbits with n ≥ 3 to the orbit with n = 2. The differences in energy between these levels .Chemistry questions and answers. The Bohr model for an atom is shown below. The energy levels, n, are designated as 1, 2, 3, and 4, which refer to how close they reside from the nucleus. n-4 n-3 n-2 n=1 Which electronic transition will result in the highest-energy emission of energy? On=2 to n=1 On=1 to n = 3 On = 4 ton = 1 On=1 to n = 4.
Emission spectrum of hydrogen. The Balmer Rydberg equation explains the line spectrum of hydrogen. A line spectrum is a series of lines that represent the different energy .
Step 1. The objective of the question is to predict the correct transition for the Hydrogen atom. Which transition for an electron in a hydrogen atom, shown in the diagram shown below, would result in the emission of photons of lowest energy? : Infinity EL - 3 1 .
A. The arrows represent the transition of electrons to different energy levels when heat is supplied. B. The arrows of W represent emission in the UV region. C. The smallest arrow of X represents a violet line in the emission spectrum. D. The arrows of Y represent emission of electromagnetic waves with higher energy than those represented by X .Chemistry. WAEC 2004. Which of the following electron transition results in the emission of energy? A. 3p to 3s. B. 3p to 4p. C. 2s to 2p. D. 1s to 2s.The energy released or absorbed during the transition from one energy level to another energy level . The Bohr Model of the Hydrogen Atom 7.29 In the Bohr model, which of the following electron transitions in a .

For a hydrogen atom, which electronic transition would result in the emission of a photon with the highest energy? 2s 3p. 7f 5d. 2p 6d. 6p 4s. Try focusing on one step at a time.
Study with Quizlet and memorize flashcards containing terms like Of the following transitions in the Bohr hydrogen atom, the _____ transition results in the absorption of the highest-energy photon. A) n = 3 → n = 2 B) n = 5 → n = 2 C) n = 2 → n = 5 D) n = 4 → n = 2 E) All transitions absorb photons of equivalent energy, At maximum, an d-subshell . This shows that the wavelength is inversely proportional to the energy: the smaller the amount of energy absorbed, the longer the wavelength. So, we look for the transition that involves the smallest energy. (Adapted from Chemistry LibreTexts) We see from the energy level diagram that the energy levels get closer together as #n# increases.
which electron transition result in the emission of energyThis movement of an electron from a lower energy level to a higher energy level, or from a higher energy back down to a lower energy level, is known as a transition. In order for a transition to occur, the energy of the photon absorbed must be greater than or equal to the difference in energy between the 2 energy levels. However, once the .

The figure shows energy level diagram of hydrogen atom. (i) Find out the transition which results in the emission of a photon of wavelength 496 nm. . The fig shows an energy levels for the electron in a certain atom which transition in a graph represents the emission of a Photon with most energy ?Energy Level and Transition of Electrons Study with Quizlet and memorize flashcards containing terms like Of the following, _____ radiation has the shortest wavelength, Of the following transitions in the Bohr hydrogen atom, the _____ transition results in the emission of the highest-energy photon., In the Bohr model of the atom, _____. and more.
which electron transition result in the emission of energy|Energy Level and Transition of Electrons
PH0 · Which electron transition results in the emission of energy? a. 3p to
PH1 · Which electron transition results in the emission of energy? a. 3p
PH2 · SOLVED: Which electron transition results in the emission of ener
PH3 · Energy Level and Transition of Electrons
PH4 · Electronic transitions and energy (video)
PH5 · Electron transitions
PH6 · Electron Transition
PH7 · Bohr's model of hydrogen (article)
PH8 · Atomic electron transition
PH9 · 3.3: Electronic Transitions
PH10 · 1.1: Electronic transitions and luminescence
PH11 · 1) which electron transition results in the emission of energy?A.